
04 Mar Implant Development Engineer
Implant Development Engineer
Paris · Full time
Research, development and industrialization of cutting edge miniaturized retinal implants that allow blind people to see again
Pixium Vision is developing innovative Vision Restoration Systems, which are active implantable medical devices intended to have blind people regain visual perception and greater autonomy. Pixium leverages the rapid advances in visual processing, microelectronics/nanoelectronics, optoelectronics neurobiology, and intelligent software algorithms in its miniaturized complex optoelectronic systems.
The Prima implant is to date the smallest artificial vision device and holds the world record for prosthetic visual acuity in patients. The implant is a 2-mm large, 30-µm thick microchip array of photodiodes and electrodes coated with biomaterials. This chip combines cutting edge semiconductor and MEMS manufacturing technologies as well as advances in bio-coatings for neurostimulation.
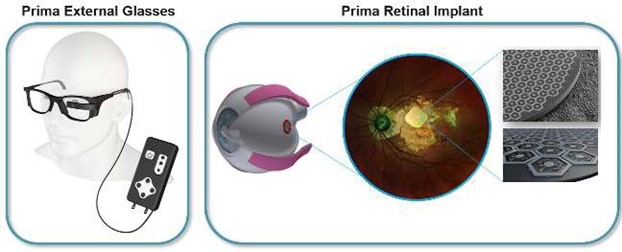
Pixium is looking for an engineer for the research, development, industrialization and production of the Prima retinal implant set.
We are looking for a passionate, scientifically skilled and hands-on technical engineer who wants to make a difference in patient’s life while developing state of the art neurostimulation microdevices within a fast pace start-up environment.
Role
Research, development, implementation, and manufacturing of the retinal implant core technologies at Pixium Vision
Responsibilities
- Through analysis of implant construction and function, identify and describe methods to test, verify and validate the implant features such as functionality and reliability,
- Perform implant test studies, monitor the tests and analyse the test data,
- Supervise technician and operator work in the framework of these studies as necessary,
- From test data analysis and synthesis, draw conclusion on the implant capabilities. Propose improvement of implant design and features as necessary,
- Document the implementation, operation, monitoring and analysis of the tests through dedicated protocols and reports,
- Support implant production as necessary,
- Accomplishment of all the above whilst ensuring compliance with the Quality System.
Minimum Qualifications/Experience
- Engineering degree or similar from a good university, engineering school or similar,
- Some experience in the Medical Device industry would be appreciated,
- Ability to understand and tackle complex technical issues, in particular related to electrochemistry,
- Experience in test bench development,
- Excellent communication skills (written and verbal) in English and one other European language.
Personal Attributes
- Analytical, methodical engineer
- Technically competent, problem solver, results-oriented
- Team player, open mindset
Position reporting to the Implant Development Manager
Please click here to apply.


Sorry, the comment form is closed at this time.